Saquinavir Unmasked: An Adrenoceptor Agonist In Disguise
Tahoe-100M revealed that Saquinavir unexpectedly mimics mechanisms such as adrenoceptor agonism, explaining clinical off-target effects discovered after decades of clinical usage.
Drugs are polypharmacological. During discovery and development, we often treat them as chemical agents that are optimized to interact with only the desired target. But in reality, they do a lot more; they interact with other parts of the biological system that is the patient.
They bind to other proteins and perturb other, unintended landmark genesets.
Many of these perturbations remain unknown for decades, even after the drug is approved for use in patients. Some of them are desirable; they are a lucky contributor to the drug action. And some are deleterious off-target effects.
Overall, these perturbations add up to become the overall phenotype of the drug and how effective it is. What if, instead of designing a drug and hoping that the polypharmacology works out, we were able to deconvolve and account for the effect of that drug on many key landmark genesets throughout discovery and development?
Gigaset, perturbative, single-cell maps like Tahoe-100M make this a reality. They provide a high-precision reference of how thousands of compounds perturb landmark genesets at single cell resolution. To deconvolve the polypharmacology of a given drug, all you need to do is to compare the post-perturbation transcriptional profile of that drug with these giant maps and see what other perturbations it mimics.
That is exactly what we did for a poorly characterized, but consequential drug: Saquinavir, a once approved HIV protease inhibitor, which was later discontinued.
By comparing transcriptional responses of Saquinavir (among many other drugs) with unknown mechanisms to well-characterized reference drugs, we created a detailed drug-drug similarity map. This approach leverages the rich transcriptional data from our Mosaic platform as a lens to quickly pinpoint drug similarities, elucidating their cellular effects (Fig 1a).
(If you want to know more about how we created these similarity maps, take a look at the postscript in the bottom of this post)
Transcriptional similarity in Mosaic data allows for drug::drug mechanism of action mapping. A) Conceptual heatmap illustrating clustering of transcriptional profiles from known and unknown drugs using Mosaic data as a way of inferring MOAs. B) Ranked plot of Mosaic transcriptional similarity scores between Saquinavir and a library of compounds. Norepinephrine and Vilanterol rank highly, indicating strong transcriptional resemblance and possible mechanistic overlap.
And that’s where surprises started to emerge from the data: Saquinavir, rather unexpectedly, mimics classic adrenoceptor agonists like Norepinephrine and Vilanterol. Classically, adrenoceptor-agnonists like Norepinephrine activate adrenergic signaling, leading to hallmark effects in cells such as increased intracellular levels of cAMP and phosphorylation of CREB (pCREB). Traditionally, Norepinephrine is clinically utilized to manage acute hypotension, highlighting its significant cardiovascular activity. Saquinavir’s unexpected resemblance to these agonists (Fig 1b) suggested potential unintended effects on cardiovascular function.
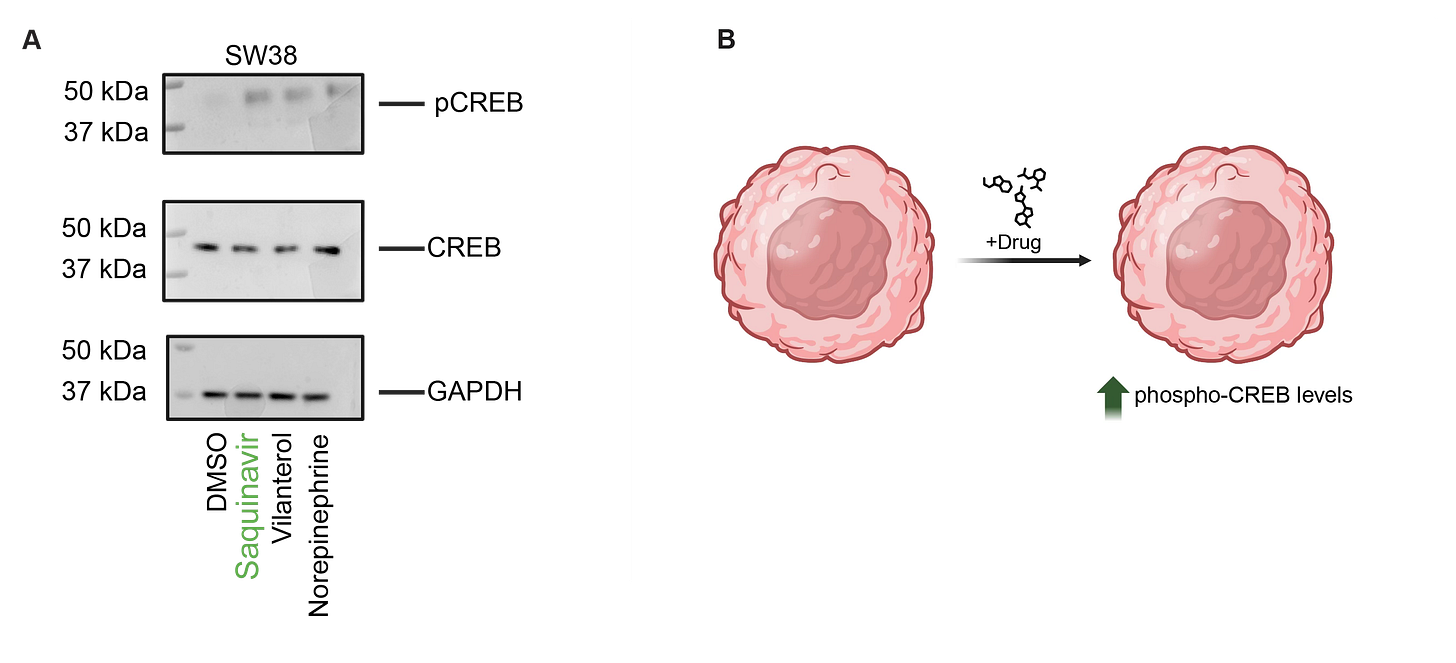
We discovered this in our Tahoe-100M similarity maps; does it stand up when tested in traditional experimental assays? The answer is yes! We treated a representative cell line with Saquinavir and, separately, with adrenoceptor agonists. We confirmed that after treatment with drugs, Saquinavir showed the same levels of upregulation of pCREB as traditional adrenoceptor-agonists Vilanterol and Norepinephrine (Fig 2a,b).
Saquinavir induces a transcriptional program similar to adrenoceptor agonists. A) Western blot showing protein expression of phospho-CREB, CREB, and GAPDH following 24hr treatment B) Cartoon summary of mechanism of action (MoA)
Things got even more exciting when we dug into the clinical history of Saquinavir: Saquinavir has been associated clinically with QT prolongation and cardiac arrhythmias, prompting an FDA safety review in 2016 (Fig 3). While QT prolongation typically results from direct ion channel inhibition, our data-driven discovery uncovered an additional mechanistic similarity between Saquinavir and adrenoceptor agonists, potentially contributing to broader cardiovascular risks through adrenergic pathway activation. This provides novel mechanistic context to documented clinical safety concerns and expands our understanding of the cardiovascular liabilities associated with Saquinavir.
FDA Drug Safety Communication on Saquinavir highlighting unknown mechanisms underlying cardiovascular adverse effects. Source.
This is remarkable! Access to Tahoe-100M allowed us to uncover off-target effects that were discovered after three decades of widespread clinical usage. Imagine what else we are missing in the pipeline of drugs that are being designed today and making their way through the clinic in the next decade.
What makes this even more remarkable is two important observations:
Despite stark differences in chemical structure, Norepinephrine, Vilanterol, and Saquinavir converge functionally through a shared transcriptional mechanism of action (Fig 4). Such revelations highlight the transformative power of transcriptional similarity in reshaping our understanding of drug pharmacology and safety.
Interestingly, Saquinavir was specifically designed as an antiviral agent to inhibit HIV protease, targeting the viral lifecycle rather than human biology. Because its development prioritized antiviral efficacy over broader human cellular responses, the unexpected adrenoceptor agonism and cardiovascular risks remained undetected.
This underscores the power of Tahoe-100M (and datasets with similar content and scale) as a new modality of data to guide discovery and design of drugs by rapidly uncovering drug effects across diverse biological pathways—revealing critical mechanistic and safety insights as well as therapeutic opportunities that traditional screens would miss.
Chemical structures of Norepinephrine, Vilanterol, and Saquinavir.
Tahoe-100M exemplifies how new modalities and scales of pharmacogenomic data advance therapeutic innovation and inform patient safety. We hope this example inspires broader applications of our data, continually driving progress toward safer and more effective treatments. Until next time.
Postscript
We mentioned that we created drug-drug similarity maps from Tahoe-100M. Here is how we did it: